Title of paper under discussion
The sensation of groove engages motor and reward networks
Authors
Tomas E. Matthews, Maria A. G. Witek, Torben Lund, Peter Vuust, Virginia B. Penhune
Journal
NeuroImage, Volume 214, 1 July 2020, 116768
Link to original paper (open access)
Overview
Researchers in Aarhus, Denmark, set out to investigate the effects of musical groove on the listening brains of musicians and non-musicians.
Short groovy musical excerpts (of medium or high rhythmic complexity) were played to the participants in an fMRI brain scanner, and they were asked to rate the music according to 1) ‘beat strength’ 2) how much it made them ‘want to move’ and 3) how much ‘pleasure’ it gave them.
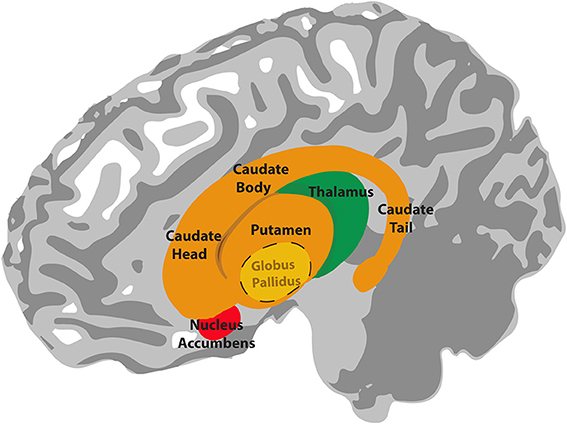
It turned out that rhythms of medium (rather than high) complexity elicited the highest ‘beat strength’, ‘pleasure’ and ‘wanting to move’ ratings, making the brain more active in motor regions associated with beat perception and expectation, and in reward/pleasure areas. Vital to both these motor and reward networks are the basal ganglia, the brain’s relay stations between emotion, movement and thought.
Introduction
The sensation of groove is defined by scientists as ” the pleasurable desire to move to music”. Deriving pleasure from music is commonly thought to stem from the “interplay between the violation and fulfilment of musical expectations”, with perhaps the most basic of musical expectations being whether a note is going to fall on a particular beat. Lead authors Tomas Matthews and Maria Witek therefore proposed that enjoyment of musical groove might involve 1) internalising the musical beat, making use of actual or imagined movement such as foot-tapping 2) predicting whether upcoming notes are going to fall on particular beats and 3) deriving an emotional reaction according to whether that expectation is violated or fulfilled. That emotional reaction is not as simple as “expectation violated = sad, expectation fulfilled = happy”; previous research shows that too little violation is boring and too much violation is unpleasurable. Hence a medium groove, for example:

should, they judged, elicit maximum pleasure – just enough violation to tickle the brain’s pleasure centres.
In the authors’ words: “the sensation of groove can be framed as the intersection of reward processing and the motor processes that underlie beat perception, with rhythmic expectations as the driving mechanism.”
Method
57 participants were each presented with a run of 36 different short groove excerpts, once inside the fMRI scanner and once outside it. Half of the groove excerpts were were of medium rhythmic complexity (taken from Afro-Cuban rhythms called ‘son clave’ and ‘rumba clave’) and the other half were of high complexity (copies of the medium grooves but with every note except the first being shifted back or forward in timing).
[For the sake of brevity I’m here concentrating on rhythm, the main focus of the paper, although the researchers also investigated the additional variable of medium vs high harmonic complexity].
While in the scanner the participants were asked to rate each groove according to how much it made them ‘want to move’. Outside the scanner they were played exactly the same playlist of grooves, this time being asked to rate each groove according to its ‘beat strength’ and how much ‘pleasure’ it gave them.
The scan images and the ratings were then all compared to look for correlations between ‘beat strength’, ‘wanting to move’, ‘pleasure’ and activity in certain parts of the brain.
Results
‘Pleasure’, ‘wanting to move’ and ‘beat strength’ ratings were all strongly correlated with one another, and all these ratings were higher on hearing the groove of medium – as compared with high – rhythmic complexity (especially ‘pleasure’ in non-musicians).
Brain activity when listening to a groove of medium – as compared with high – rhythmic complexity is shown here:
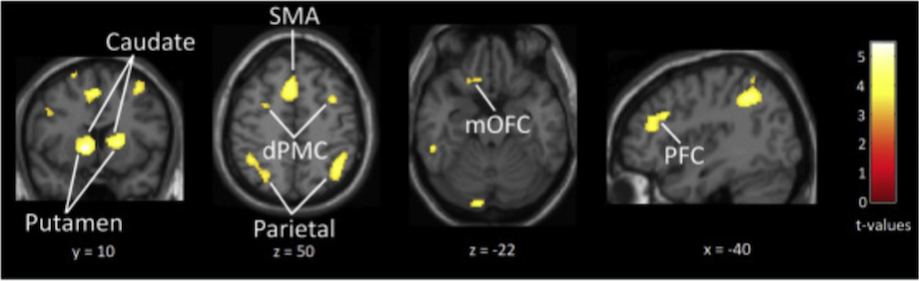
This image shows greater activity in reward-related regions including the nucleus accumbens (NAcc), caudate and medial orbitofrontal cortex (mOFC) as well as in beat-related regions such as putamen, supplementary motor area (SMA), prefrontal cortex (PFC) and parietal cortex.
Further result analysis, relating ratings to brain activity, showed that ‘pleasure’ and ‘wanting to move’ were both associated with greater activity in the nucleus accumbens, caudate, putamen and medial orbitofrontal cortex, and that although these ‘pleasure – brain activity’ and ‘wanting to move – brain activity’ associations were often very similar (overlapping) it was sometimes possible to tease them apart, with ‘wanting to move’ a stronger predictor of activity in the putamen, caudate and mOFC.
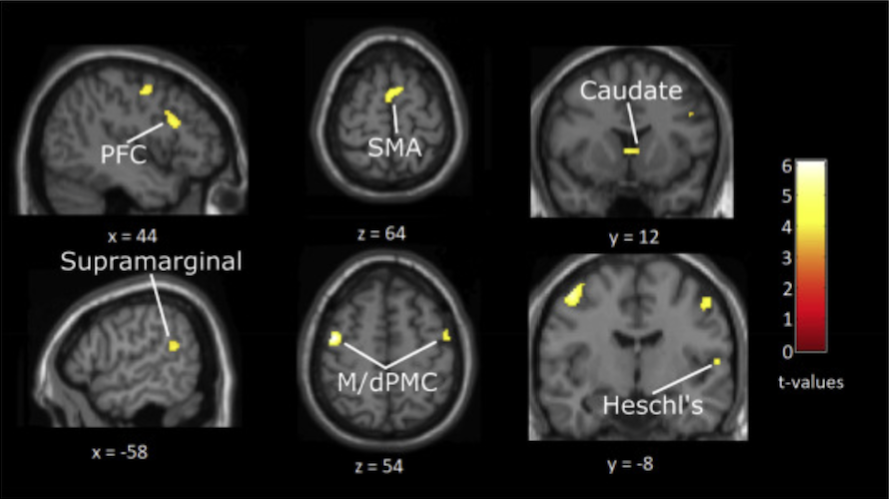
Brains of musicians compared with non-musicians, both listening to grooves of medium rhythmic complexity, revealed extra activity in auditory areas (right Heschl’s gyrus and left posterior superior temporal gyrus) and in beat-related regions such as putamen, right frontal cortex, caudate, motor cortex and supplementary motor area:
Discussion
Matthews and Witek et al proposed a model to explain their findings whereby beat -generating regions (putamen, SMA and premotor cortex) produce an internal representation of the beat in the groove being listened to. These beat-generating regions then interact with rhythmic-expectation regions (caudate, prefrontal and parietal cortex), which constantly compare the expected rhythm to what is actually heard. Information from these two networks then passes to the affective regions (nucleus accumbens, mOFC) which generate responses of ‘pleasure’ and ‘desire to move’. Grooves of medium, as opposed to high, rhythmic complexity activate these networks more effectively because “they are regular enough to allow for internal beat generation, but also contain syncopations that challenge this regularity and thus engage expectation processes leading to the pleasurable desire to move”. As for the extra ‘lighting up’ in musicians’ brains compared to non-musicians – the authors suggest that “musical training may strengthen these [higher level] expectations and the brain networks that support them”.
Coda